I just added a new Page to the static content of our site showing samples of the endbands (headbands) that we sell.
Measuring US Display Mould Spacers
In order to make an 18 point spacer for use in a US Monotype Display Mould, I first have to determine the dimensions required. I set up a spreadsheet for recording the data and measured the dimensions of all the spacers I had.
My first observation is that, except for the thickness, there is a lot of variation in the dimensions, with differences of up to 0.010″ from one spacer to the next. This will mean making a replacement spacer should be relatively easy in terms of machining the outline of the part.
My other observation is that there seem to be two styles of blade and correspondingly two sizes of spacers:
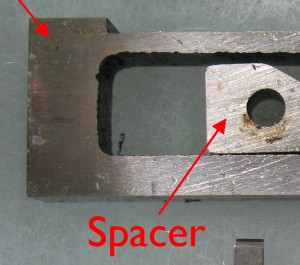
On the left the blade has a thick end, about 5/16″, and the spacer is about 7⅞″ long. On the right, the blade has a thinner end, about 3/16″, and the spacer (not shown) is 11/16″ long with most of the extra length to the left of the hole. In addition to determining the space between the two type blocks, the spacer also acts as a stop limiting how far the blade can open. Installed and properly adjusted in the caster, this stop is never used (the sizing mechanism limits the mould blade opening), but when the mould is out of the caster, this spacer holds the blade from coming out completely. Ideally, the blade should not be able to pull out enough to disengage the nick wire in the left type block from the nick machined in the side of the blade. If the blade comes out further than this, there is the possibility of damage when closing the blade again if the nick wire improperly reengages with the blade nick. The only thing holding the nick aligned is the front blade cover (shoe).
If a short spacer is used with a thick-end blade, or a long spacer with a thin-end blade, this stop seems to work properly, keeping the nick engaged. But as I mentioned in my previous post about blade kits, the spacers are not serial numbered and can become interchanged between kits, leading to the inappropriate use of a short spacer with a thin-front blade. Several of my blade kits are like this. Conversely, a long spacer combined with a thick-end blade will limit the blade opening; this is unlikely to be a problem for small sizes, but 36-point display type requires the mould blade to open to 48 points, and the long spacer might prevent this.
Note also the rough finish to the opening in the blade, again indicating that high precision machining of the spacer is unnecessary.
The thickness of the spacer, on the other hand, is very important, as it determines the body size of the type. The spacer holds the left and right type blocks apart during mould assembly, but once everything is together and both type blocks have been tightened down, the spacer really only acts as a blade stop. I measured the thickness of all the spacers I had and correlated this to their nominal size, and found that they are about 0.36% thicker than their nominal size. This is to allow for the shrinkage of the type as it cools from the type metal’s solidification temperature to room temperature. The thickness measurements were done with a micrometer, which measures to 0.0001″ (a little better than 1/100 of a point), but I found that the thickness of individual spacers varied by up to ±0.0003″ from the exact 0.36% oversize. This variation did not correspond in any way with the serial number of the mould, but as I mentioned, the spacers can be interchanged between kits.
Just in case this represents measurement error, I will re-measure the thicknesses by comparing each spacer thickness against a set of gauge blocks. This will be done using the following setup, with which I was checking the thickness consistency and parallelism of the spacers:
The dial indicator is clamped to the head of a height gauge, and the latter is adjusted to a height that gives a non-zero reading on the indicator and then locked so it does not shift. Everything is set on a granite surface plate. This does not give an actual thickness measurement, but by sliding the spacer around under the tip of the indicator, I can see how much the thickness varies. The small graduations on the indicator are 0.0001″ and in this case the thickness varied by a bit less that that. By replacing the spacer with gauge blocks, I can obtain an exact (as exact as the tolerance of the blocks) thickness.
I also still have to measure the hole size and position. One other observation to simplify my machining work is that this spacer does not rub on anything nor is it subjected to any impact or localized pressure. As a result it does not have to be hardened so I can make my new spacer from low-carbon steel, which will be easier to machine than a hardenable grade of tool steel.
Up, Down, What’s the difference?
My recent post about setting up to cast 18 point display type got up and down mixed up. When casting 18 point on an English mould with an American display mat holder, the mat must be lowered, not raised as I had stated. There is a mat holder insert kit to accomplish this, but it is the one insert kit that I don’t have.
I have added corrections and an addendum to the original post explaining the oopsie.
Bald eagle sighting near Kitchener?
On Tuesday I was driving along highway 401 where it crosses the Grand river in south Kitchener when I saw the silhouette of a very large bird flying south of the highway.
I only saw it for a second or two before the view was cut off by the top of the windshield (and besides, I was driving), and saw it mostly in silhouette against dark clouds. Just before it slipped out of sight, though, I saw that it had a white head.
So I’m pretty sure it was a bald eagle, the first time I’ve seen one not in captivity. I was mildly surprised to see one so close to the city, but there have been other sightings elsewhere along the Grand river in the Kitchener area.
Glycerin Specific Gravity
Today I used my pycnometer and precision scale to measure the specific gravity of the glycerin which we sell and which I use when I’m trying out recipes for home-made composition rollers.
I started taking a video of this procedure but my camera battery ran out of juice before I was done, and the spare batteries were hiding in some Wii controllers and also pretty much drained. I didn’t want to wash up the equipment and restart another day, and I didn’t want to leave everything out either, so I will have an incomplete video of the process posted later. It turns out that you can only watch someone filling a pycnometer, drying its outside, and weighing it two or three times before it gets tiresome anyway.
The only thing missing from the video is the extra step, when filling the pycnometer with glycerin, of rinsing the overflow off the outside of the bottle before trying to wipe it dry. This ensures there is no film of glycerin on the outside of the bottle adding to its weight.
In any case, the pycnometer held 9.934g of water or 12.505g of glycerin (these are weights, not mass, because there has been no compensation for the buoyancy from the atmosphere), yielding an apparent specific gravity of 1.2588 for the glycerin. Interpolating into a table of apparent specific gravities of water/glycerin mixtures gives the value of 98.1% glycerin, and 1.9% water by weight. Contrary to what I had previously stated, the glycerin bottle does state that it is 99.7% pure. We’ve had this bottle for a while, and it is now only half full, so some water has been absorbed from the air over the years, explaining why I found more than 0.3% water. Further evidence of this is that the bottle was slightly collapsed from the water being drawn out of the air in the bottle.
18 Point Frustration
I have some display casting to do in 18 point on my Monotype Composition Caster, but I’m having a tough time finding a set of accessories that will work together to accomplish this.
My original intent was to use my 18 point display mould made by Monotype Corp. in England, and my display matrix holder for using English display moulds with American-style display matrices (part code 49A). This was my preferred route because this mould is in excellent condition. But, try as I might, I’m utterly unable to find this mat holder amongst my casting equipment.
Failing that approach, one alternative would be to use my American-made display mat holder with the English mould. The problem here is that for all display sizes other than 12 and 36 point, the position of the mould cavity is different on American and English display moulds. In particular, for 18 point, the English mould cavity is 3 points higher lower (in terms of matrix orientation) than the American mould cavity. The American mat holder has 4 sets of interchangeable inserts to modify matrix alignment, but none of these is designed to raise the mat—they all lower the mat (typically for casting all caps on a size body smaller that the face’s size) by varying amounts.

American mat holder insert sets. This is not a full selection of all 4 sets as there is one duplicate here.
It is plausible that I might be able to modify one of these sets to raise the mat by 3 points (or, to match the motif of the genuine sets, 0.050″, about 3.6 points), but one piece, in the upper left of each set in the photo, would have to be made longer so I might have to make this from scratch. This is not something I would look forward to because this part has a fairly complex shape. Maybe the lugs underneath can be trimmed to the same effect, maybe not. But that’s not really relevant because I got up and down confused.
The third approach would be to go completely with the American system, using an American mould and American mat holder. This would seem like a sensible way to have started, but my American display moulds are in sad shape: they are dirty, heavily used, and have unknown amounts of wear.
The American moulds work with interchangeable mould blades for the various body sizes. One basic mould (type U) is used for 24 to 36 point type, while the other (type T) is used for 12 to 18 point. I have never encountered any mention of display sizes between 18 and 24 so if such exists, I don’t know whether the T or U mould is used. The type T and U moulds actually only differ in one part, the right type block, which determines the position of the nick-side of the mould cavity (to the operator’s left, defining the bottom face of the type). All other size changes are done by positioning the right type block (defining the top face of the type). The actual casting size is determined by a mould blade kit which consists of 6 parts:
 These parts are custom fitted to a particular mould, and you can see the serial number of the mould marked on the pieces. The spacer holds the left and right type blocks the correct distance apart and is the one kit part that is not tied to a particular kit or mould serial number—it is only specific to the body size. The spacer also limits how far the blade can open (to widen the type).
These parts are custom fitted to a particular mould, and you can see the serial number of the mould marked on the pieces. The spacer holds the left and right type blocks the correct distance apart and is the one kit part that is not tied to a particular kit or mould serial number—it is only specific to the body size. The spacer also limits how far the blade can open (to widen the type).
The blade stop, top blade, and main blade are fitted together to the mould so that when the blade is closed, its end (both main and top) are just flush with the front surfaces of the type blocks. This exactly ejects the type without the mould crossblock rubbing on the blade. The top and main blades are also fitted to each other so their ends are flush when the blade is partway open for casting type.
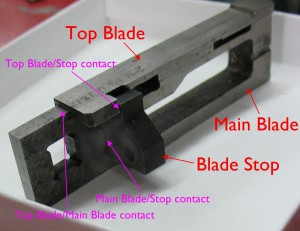
The top and main blade with blade stop, positioned as if installed in the mould, to show which surfaces determine the blade position. The back of the blade stop can be ground to adjust the overall blade position in the mould.
I have quite a few kits:
 …but only two of these are complete and have the matching mould. Worse yet, here is my only 18-point mould blade kit:
…but only two of these are complete and have the matching mould. Worse yet, here is my only 18-point mould blade kit:
 The spacer and back cover are missing! As it turns out the back cover for an 18 point T mould and 30 point U mould are almost identical. There does not appear to be any close fitting involved (in a mould assembled with no front cover the blade can be visibly shifted up and down) so in a pinch I could use the back cover from a 30-point kit. The spacer is the critical part and since this is the only 18-point kit I have I can’t borrow the spacer from another kit.
The spacer and back cover are missing! As it turns out the back cover for an 18 point T mould and 30 point U mould are almost identical. There does not appear to be any close fitting involved (in a mould assembled with no front cover the blade can be visibly shifted up and down) so in a pinch I could use the back cover from a 30-point kit. The spacer is the critical part and since this is the only 18-point kit I have I can’t borrow the spacer from another kit.
So it looks like I might be making an 18-point spacer for an American display mould. The shape of the part is fairly simple and does not require extraordinarily precise machining work, except for its thickness, which must be accurate (and the faces parallel) to 0.0001″ to get properly-sized type.
In the meantime, I can only hope that my 49A display mat holder will pop up, saving me all this trouble!
Addendum
As often happens concerning the alignment of American display mats, I had my up and down reversed. The 36- and 12-point mats have the same alignment in both systems. Sizes smaller than 36 that use the Type U mould (e.g. 30 and 24) are lower in the American mould than in the English one, while sizes larger than 12 that use the Type T mould (e.g. 14 and 18) are higher in the American mould than in the English one. As a result, the 18-point mats I want to use in the American holder must be lowered by 3 points to fit the English mould. This could be accomplished by using the first replacement mat holder insert set (41A19, 20, 21) which lowers the mat by 0.050″ except that is the one set I don’t have. Even though this set is a standard accessory to the mould it would be just as much trouble to make as a custom set to raise the mat.
This still pretty much leads me into the third option.
Fixing up a laboratory scale
Several years ago I purchased at an auction a laboratory scale for doing precision weighing. Up until now it has sat in a corner collecting dust, covered with auction stickers and missing one cover.
I recently undertook to clean and fix it up. The scale is a Precisa 125A which has a range of 125g (about 4 ounces avdp) and a readability of 0.1mg (from later tests, that’s about the weight of a speck of dandruff).
The weighting platform is enclosed in a glass cabinet to keep it clean and also so air currents don’t disturb the weighing. This cabinet was missing its top cover although the handle for the cover was included. The remaining glass and cabinet frame had residue of masking tape adhesive, dust, and a sticker from the auction.
 Some work with lighter fluid and a plastic scraper blade, followed by glass cleaner, gave me 3 clean panes.
Some work with lighter fluid and a plastic scraper blade, followed by glass cleaner, gave me 3 clean panes.
 I cut a piece of 1/16″ glass to fit the top. The rough cut was a bit too wide so I used a diamond cutting disk in my Dremel to remove a bit of the width. Then I polished all the edges smooth and round using a coarse diamond sharpening stone with soapy water to lubricate the work and wash away the glass dust.
I cut a piece of 1/16″ glass to fit the top. The rough cut was a bit too wide so I used a diamond cutting disk in my Dremel to remove a bit of the width. Then I polished all the edges smooth and round using a coarse diamond sharpening stone with soapy water to lubricate the work and wash away the glass dust.
I set up my Dremel drill press with a ⅛″ diamond grinding bit to drill the holes.
 The diamond sharpening stone also turned out to be just the right height to support the scrap board that would hold the glass for drilling. The handle for the top cover is sitting on the glass, and needs two 4mm holes 4cm apart. I have a video of me drilling the holes and enlarging them to 4mm.
The diamond sharpening stone also turned out to be just the right height to support the scrap board that would hold the glass for drilling. The handle for the top cover is sitting on the glass, and needs two 4mm holes 4cm apart. I have a video of me drilling the holes and enlarging them to 4mm.
Here is the top cover with the handle attached. I used two tiny screws and washers recovered from dead computer equipment to hold it on. I also placed a narrow strip cut from PVC electrical tape along one edge to act as a bumper and dust seal where this cover meets the front glass of the cabinet. I use my heat gun to eliminate any stretch in the tape (from unrolling it) before applying it to the glass in the hopes that this will reduce the tendency to peel. There are already similar strips on the two side covers.
 Here is the scale all cleaned up with the cabinet assembled.
Here is the scale all cleaned up with the cabinet assembled.
 And here it is weighing a short length of clear plastic (likely polypropylene) filament, weighing in at 7.2 milligrams. I couldn’t find anything in my shop that I could see and pick up that weighed less than about 5mg. I suppose I should have tried a shorter length of the filament.
And here it is weighing a short length of clear plastic (likely polypropylene) filament, weighing in at 7.2 milligrams. I couldn’t find anything in my shop that I could see and pick up that weighed less than about 5mg. I suppose I should have tried a shorter length of the filament.
 Now that I have a precision scale I can try using my pycnometer to measure the water content of our glycerin.
Now that I have a precision scale I can try using my pycnometer to measure the water content of our glycerin.
Another project has now suggested itself: a dust cover for this scale, including pockets for the power cord and manual. While I’m at it I should also make covers for my microscope and the binocular scope mounted on my lathe.
A pycnometer
That’s ‘pyc’ sounding like an ice ‘pick,’ ‘n’ as you would expect, and the rest rhymes with ‘barometer’ with the ‘nom’ being the stressed syllable.
This is a fancy name for a container which can be filled to a very reproducible volume of liquid (there are also pycnometers for powder). Even though the actual volume is only roughly known, and varies from one pycnometer to another apparently identical one, the fixed volume is still sufficient to measure the specific gravity of liquids.
This used one I bought holds about 10ml (⅓ of a fluid ounce). The stopper has a fine hole drilled through it up to the top. You fill the bottle to the brim, and insert the stopper. As the ground glass joint of the stopper seats, the last bit of excess liquid is forced out through the hole, leaving you with exactly the same volume of liquid in the bottle each time. Of course you have to clean and dry the outside of the bottle before weighing it.
Essentially, you weigh the empty pycnometer, weigh it filled with a reference liquid (almost always pure water), and weight it a third time filled with the liquid whose relative density you want to determine. A fairly simple calculation, which includes the current atmospheric pressure since the buoyancy from the air affects the apparent weight, yields the relative density of the tested liquid as compared to the reference liquid. When the reference liquid is pure water, this relative density is called the specific gravity. Because the result is actually the ratio of two densities, the facts that the volume of the pycnometer is not known accurately, and that the local gravitational force might not be known exactly (it varies from location to location) don’t affect the result. Details on the calculations can be found in this Wikipedia article.
One liquid I want to test with this is the glycerin we sell. Glycerin almost invariably contains at least some water since it is so hygroscopic, but the stuff we sell is not labelled as to water content. It would be useful to know this information, not only to keep our customers informed, but also since this can affect the recipe used for making composition (a rubbery material made of gelatin+glycerin+sugar+water) rollers for printing presses. Given the specific gravity of a mixture of glycerin and water, the fraction of water can be determined by consulting the appropriate reference table.
I’m just fixing up the precision weigh scale I have (which weighs to the tenth of a milligram), and once that it ready I can test our glycerin.
Preparing to cast some type
Someone has asked me to cast some type to fill out a font they already have. For reasons unknown, he has almost no capital S and is also short on capital C and T. He mailed me a sample type of each to ensure that the new type I cast would match the alignment of his existing type. Although there is a standard alignment for most fonts, this is not always followed and it is essential for new type to duplicate the alignment of the old type it will be mixed with, even if this alignment is non-standard.
As a preliminary to doing this casting work, I sat down for a few minutes to measure the size of the samples he had sent—something else I should also match. The result is this absolutely riveting video of me sitting at a table with a micrometer and slide rule. The video has been sped up at times where I have nothing to say about the process.
I found to my surprise that the type was cast 1 to 2 points narrower that the standard size. According to the markings on the matrices, they should have been 11, 10.5, and 11 points (0.1521″, 0.1452″, and 0.1521″) but were instead 0.1315″, 0.1187″, and 0.1422″ for the S, C, and T respectively. The faces of the type overhung to such an extent that the combinations “CT” and “ST” could not be set without a copper or brass space between the letters, or perhaps filing off some of the beard on the overhang.
The face in question is 18 point Swing Bold (Lanston Monotype #217). This is a script-style face with the lowercase letters all joining to each other as an imitation of handwritten text. The capital letters don’t join to the lowercase, though. The face also includes a lead-in stroke to use at the start of lowercase words to clean up what would otherwise be an abrupt start to the stroke (where it would normally join the previous letter). The specimen pages don’t show these lead-in strokes in use, though. Although they provide nice finish to the words, they can’t be used before the second letter of a capitalized word. Doing so would generate too much visual space between the capital and next letters. Perhaps these narrow-cast capitals are intended to be used with the lead-in stroke.
The following examples are approximations made from a scan from the specimen page for this face. The crowding of the S does not necessarily correspond to the width reduction of the type samples I have, but this shows the general effect.

This is a word taken from the text sample on the specimen page. Note how the lowercase letters join, but the start of the t is rather abrupt since it is right at the edge of the type body.

This is the effect you would get with the S cast narrow, so it can be closer to the t. Although some of the space is gone, there is still a rough transition from the S to the t.

This is what the word would look like if the lead-in stroke were added to the t and using a normal-width S. The start of the stroke looks much better, but the large space between the S and t make this look like two words (what is an “s tyle”?).
Whatever the reason these are cast narrow, I’ll have to verify that it wasn’t just a fluke that the samples I received were some rare narrow-cast letters from a case of otherwise standard width ones.
More home-made Monotype Caster parts
Someone had asked me for some parts for their Monotype Compositon Caster. Like me, he has the pump latch mechanism but he’s missing the modified pump spring rod and spacer sleeve, so he asked me to make a set for him, following the model from when I made my own a year and a half ago (see here and here).
New parts are in general not available. They are not being made, and it is a very long shot to get some new-old-stock items from places like the Type Archive in London. As a result, caster parts are often obtained by stripping down surplus casters which have somehow been saved from a trip to the scrapper. The pump latch mechanism, as well as most of the other parts for display casting, are distinctively recognizable for what they are, and generally take up little storage space. The spacer sleeve and extended pump rod are less distinctive, and the rod is bulky to store, and people don’t think of these as parts that break and need replacement, so they are more likely to be scrapped or at least lost in a jumble of miscellaneous parts.
So it is not uncommon to be in the situation of having the pump latch but not the pump spring rod modifications required to use it. To get around this problem when I ran into it, I made my own parts. Now, I’ve made another set of the same parts for someone else in the same predicament.
I have posted a video of me explaining their purpose and test-fitting them to my own caster. It was a good thing I did this because at first, the rod extension would not screw all the way onto the standard spring rod. The female threaded hole on the extension was too shallow, and also was not counterbored to allow for the unthreaded area on the end of the rod. The video wasn’t turning out well either as I found myself babbling aimlessly without some notes to work from.
The video is less than ideal… For part of it I’m showing the relevant page from the parts manual and things are pretty hard to see because the video is only 320×240 resolution, and by the time I noticed how the page would look I was done editing the video. The editor I am using (VSDC video editor) does not allow you to change the basic resolution of a project; you have to choose it when you create the project and live with it. There was quite a bit of cutting and splicing of the video clips and there was no good way to copy all those editing decisions to a new higher-resolution project.
There is one magic spot where my hand vanishes from the picture, only to come in immediately from the side again.
That strange facial expression where I seem to be peering just to the side of the camera is because I had my laptop there acting as cue cards.
One other note about the video: I mention using a temporary thread locking compound when joining the extension to the spring rod. This is so that you don’t later find the extension left behind if you remove the rod (the extension by itself would be difficult to remove from the caster because it would be almost completely inaccessible with any form of wrench). Something like Loctite 248 (the blue stuff) would to the trick. I don’t recommend a permanent thread locking compound (like Loctite 263 red) because then you would never get the parts apart. Also, only use the thread locking compound to join the rod to the rod extension. Do not use it anywhere else, particularly where the rod screws into the rod eye below the upper crosshead!